Enolates as nucleophiles
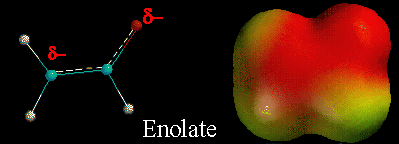
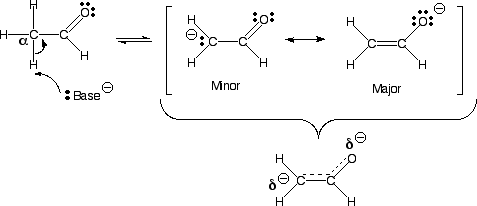
A) Enolates are resonance stabilized, with a partial negative charge on carbon and oxygen.
B) Enolates are nucleophiles, so they could react at either the carbon atom or oxygen atom. The partial negative charges give them the opportunity to react at either the carbon or oxygen.
C) Reaction at the carbon atom gives the final product a C=O bond, while reaction at the oxygen atom gives the final product a C=C bond. However, C=O bonds are stronger than C=C bonds, so the motive is to react at the carbon atom with most electrophiles.